Vinyl ethers undergo homopolymerization via a cationic mechanism.
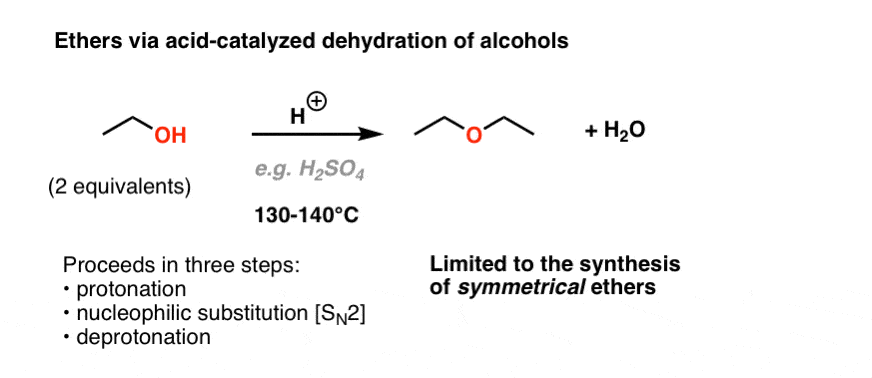
Vinyl ether synthesis.
Methyl vinyl ether also participates in 4 2 cycloaddition reactions.
Use of ethyl vinyl ether in the synthesis of glutaraldehyde.
The alkene can be deprotonated at the vinyl carbon adjacent to the oxygen.
Vinyl ethers 1 x ch 2 a o can be very effective addition fragmentation chain transfer agents.
It is also important that r is a good radical leaving group.
Organic letters 2018 20 15 4507 4511.
The optimized protocol operates under.
They are increasingly used in radiation curing systems because of a lower toxicity profile than the commonly used acrylic monomers.
Furthermore an experimentally simple stereoselective synthesis of of vinyl ethers is delineated using the same catalyst system.
The use of a p n ligand enables a highly regioselective ni catalyzed hydroalkoxylation of 1 3 dienes.
Synthesis of macrocyclic poly 2 chloroethyl vinyl ether s die makromolekulare chemie rapid communications 1991 living cationic polymerization of 2chloroethyl vinyl ether.
Merlic department of chemistry and biochemistry university of california los angeles 607 charles e.
Soc 2003 125 4978 4979.
The alkene portion of the molecule is reactive in many ways.
Methods for vinyl ether synthesis david j.
Vinyl ethers undergo radical initiated copolymerization in the presence of specific monomers such as maleates fumarates and acrylics.
8 21 227 228 the mechanism for chain transfer is shown in scheme 9 for the case of α benzyloxystyrene 15 the driving force for fragmentation is provided by formation of a strong carbonyl double bond.
A highly regio and stereoselective syntheses of α halo enamides vinyl thioethers and vinyl ethers with aqueous hydrogen halide in two phase systems.
Ethyl vinyl ether is made by reaction of acetylene and ethanol in presence of a base.
Young drive los angeles ca 90095 1569 usa.