When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc.
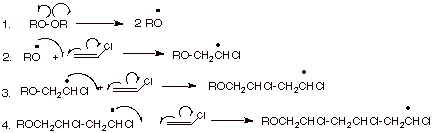
Vinyl chloride radical polymerization.
The resulting polymers exhibited precise chemical structures predetermined molecular weights and.
Physical phenomena of polyvinylchloride parti cle formation and reactant species distributions in phas s during poly merization.
Following its generation the initiating free radical adds nonradical monomer units thereby growing the polymer chain.
Pvc is used in the manufacture of numerous products including packaging films and water pipes.
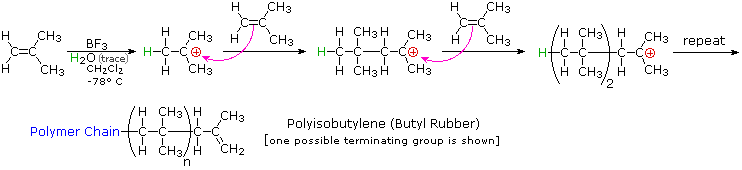
In this work we have developed a versatile and highly efficient initiation approach based on thienyl chloride derivatives with readily available starting materials for the living cationic polymerization of vinyl ethers.
Poly vinyl ether s pves are useful materials of different applications.
Return to level four directory.
Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc.
Now for free radical polymerization of ethacrylic acid.
This polymerisation reaction proceeds by a free radical mechanism.
Relevant mechanisms involved in the heterogeneous free radical polymerization of vinyl chloride have been identified including elemen tary chemical reactions.
Currently pvc is only being prepared on an industrial scale by free radical polymerization frp.
Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer.
Free radicals can be formed by a number of different mechanisms usually involving separate initiator molecules.
Additives are used to modify the properties of polyvinyl chloride to make it more useful.
A comprehensive reactor model for batch and semi.
About 13 billion kilograms are produced annually.
Chemical and physical methods were used to observe irregular structures such as branching.
Polymerization of vinyl chloride vc was studied.
It is currently used in various applications ranging from packaging to construction.
Natural evolution of hcl from vc occurred in the polymerization.
Vinyl chloride plus others.
Poly vinyl chloride pvc is one of the more consumed polymers worldwide due to its general versatility and low cost.
Free radical polymerization frp is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks.
Polyvinyl chloride is a white rigid quite brittle solid.
Second only to pe in production and consumption pvc is manufactured by bulk solution suspension and emulsion polymerization of vinyl chloride monomer using free radical initiators vinyl chloride ch 2 chcl is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst it is a carcinogenic gas that must be handled with.